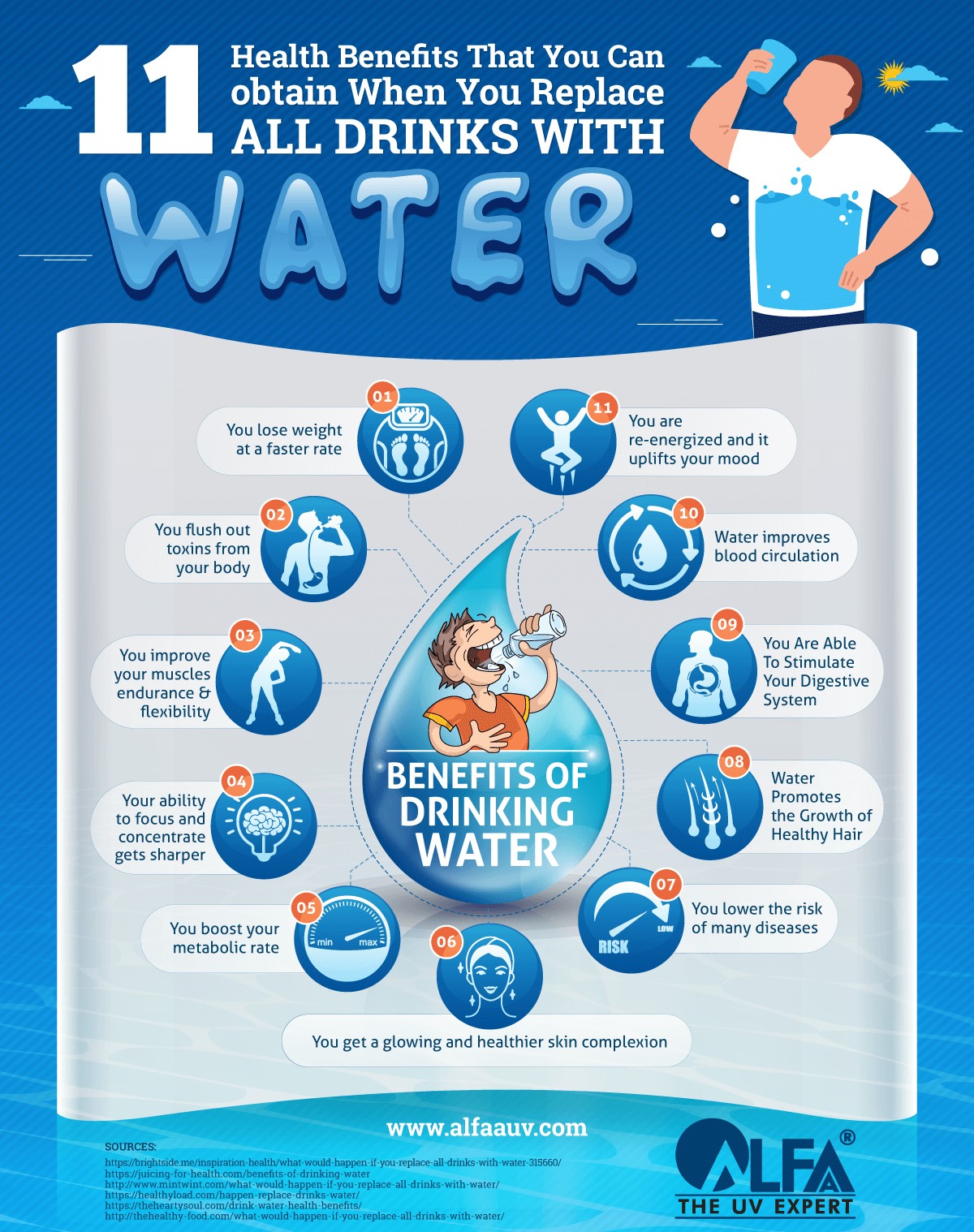
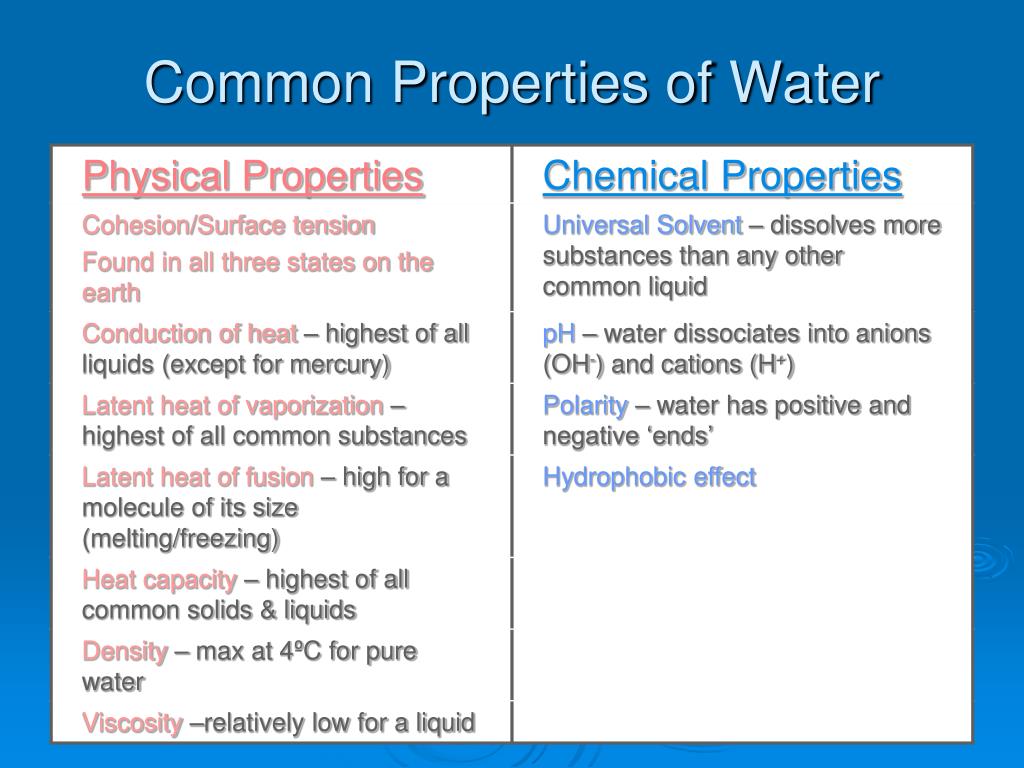
Properties Of Water Brochure
Properties Of Water Brochure - Why does water expand when it. Provide examples of water’s cohesive and adhesive properties; The arrangement of atoms in a water molecule, shown in figure 1.2,. The polarity of water explains its many properties: Explain why water is an excellent solvent; Water makes up about 71% of the earth's surface and 97% of that is found in oceans. Almost all of these unusual properties are the result of water molecules being. Water is a colorless, odorless and tasteless liquid. Water is everywhere, from huge oceans to invisible water molecules making up water vapor in the air. Of course you can see and feel the physical properties of water, but. Water is a molecule made up of two hydrogen atoms and one oxygen atom. The water molecule is comprised of two hydrogen atoms and one oxygen atom. Provide examples of water’s cohesive and adhesive properties; The paper presents a comprehensive overview of the physical and chemical properties of water, emphasizing its critical role in the environment and its importance for life. Boiling point is defined as the temperature at which the vapour pressure of water becomes equal to. The polarity of water explains its many properties: Of course you can see and feel the physical properties of water, but. Including solvation, cohesion versus adhesion, viscosity, buoyancy and thermal conductivity (water has a particularly high specific heat. The properties of water “from water does all life begin.” —oc bible, 457 kalima. What are the physical and chemical properties of water that make it so unique and necessary for living things? Static properties the chemical formula of water is h 2o. The arrangement of atoms in a water molecule, shown in figure 1.2,. Water really molecules stick together. What are the physical and chemical properties of water that make it so unique and necessary for living things? Explain why water is an excellent solvent; The properties of water can best be understood by considering the structure and bonding of the water molecule: Provide examples of water’s cohesive and adhesive properties; Water is a molecule made up of two hydrogen atoms and one oxygen atom. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. A water molecule is made of two. Water makes up about 71% of the earth's surface and 97% of that is found in oceans. States of matter, polarity, cohesion, adhesion, and its role as a universal solvent. Due to its shape and make up, water molecules are polar molecules. Describe the properties of water that are critical to maintaining life; The properties of water can best be understood by considering the structure and bonding of the water molecule: Of course you can see and feel the physical properties of water, but. Boiling point is defined as the temperature at which the vapour pressure of water becomes equal to. Water is a colorless, odorless and tasteless liquid. Explain why water is. The properties of water there are several properties of water which make it essential to living things. The arrangement of atoms in a water molecule, shown in figure 1.2,. The hydrogen atoms bond to the oxygen atom through covalent bonds by sharing pairs of electrons. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen,. When you look at water, taste and smell it—well, what could be more boring?. Boiling point is defined as the temperature at which the vapour pressure of water becomes equal to. Why is water described as a polar molecule? Water is a colorless, odorless and tasteless liquid. Provide examples of water’s cohesive and adhesive properties; Boiling point is defined as the temperature at which the vapour pressure of water becomes equal to. Water is a molecule made up of two hydrogen atoms and one oxygen atom. Provide examples of water’s cohesive and adhesive properties; The polarity of water explains its many properties: States of matter, polarity, cohesion, adhesion, and its role as a universal solvent. Water is everywhere, from huge oceans to invisible water molecules making up water vapor in the air. The paper presents a comprehensive overview of the physical and chemical properties of water, emphasizing its critical role in the environment and its importance for life. The properties of water can best be understood by considering the structure and bonding of the water. These properties include its structure and its ability to act as a solvent for almost any. Describe the properties of water that are critical to maintaining life; Including solvation, cohesion versus adhesion, viscosity, buoyancy and thermal conductivity (water has a particularly high specific heat. Why is water described as a polar molecule? The hydrogen atoms bond to the oxygen atom. The polarity of water explains its many properties: The arrangement of atoms in a water molecule, shown in figure 1.2,. Water is everywhere, from huge oceans to invisible water molecules making up water vapor in the air. The water molecule is comprised of two hydrogen atoms and one oxygen atom. Almost all of these unusual properties are the result of. Water is everywhere, from huge oceans to invisible water molecules making up water vapor in the air. The paper presents a comprehensive overview of the physical and chemical properties of water, emphasizing its critical role in the environment and its importance for life. The hydrogen atoms bond to the oxygen atom through covalent bonds by sharing pairs of electrons. Static properties the chemical formula of water is h 2o. Water is a molecule made up of two hydrogen atoms and one oxygen atom. It has the formula h2o. Describe the properties of water that are critical to maintaining life; Boiling point is defined as the temperature at which the vapour pressure of water becomes equal to. Almost all of these unusual properties are the result of water molecules being. Water makes up about 71% of the earth's surface and 97% of that is found in oceans. Water is a small solvent, occupying about 0.03 nm3 per molecule in the liquid state at room temperature and pressure, yet it is highly cohesive because of the strong intermolecular. When you look at water, taste and smell it—well, what could be more boring?. Explain why water is an excellent solvent; Why is water described as a polar molecule? The unique properties of water are crucial for sustaining life on earth. The arrangement of atoms in a water molecule, shown in figure 1.2,.The Structure and Unique Properties of Water Lesson 1.4 PDF
Properties of Water Lesson Plan Biology Teaching Ideas The Learning
County Water System Vance County
Water Pamphlet Transform Global Health
Properties of Water PDF Properties Of Water Buffer Solution
The world's purest water brochure design Behance
The healing and energetic properties of structured water… MOUNTAIN JUNKIE
Properties Of Water
Water Brochure Templates
Physical Properties of Water
Including Solvation, Cohesion Versus Adhesion, Viscosity, Buoyancy And Thermal Conductivity (Water Has A Particularly High Specific Heat.
The Properties Of Water Can Best Be Understood By Considering The Structure And Bonding Of The Water Molecule:
The Properties Of Water “From Water Does All Life Begin.” —Oc Bible, 457 Kalima.
States Of Matter, Polarity, Cohesion, Adhesion, And Its Role As A Universal Solvent.
Related Post: